Written by Kim O’Brien Root
—
[dropcap]Probably one of the most common medical procedures done today is the IV—a way to administer fluids or medication into the veins through a thin plastic needle placed in the arm or hand.[/dropcap]But although an IV seems simple enough, getting one carries a risk that patients aren’t always aware of. If the needle pokes through the vein, it can cause something called infiltration—when the fluid leaks into the surrounding tissue. The results can be traumatic.
The current practice for spotting infiltrations—as well as extravasations, when caustic medications such as chemotherapy leak—is for a nurse to constantly check the IV site. But the method is not always foolproof. If the infiltration goes on
for too long, a patient can end up with
tissue or nerve damage, sometimes requiring amputation.
“It’s really a big problem,” says Dr. Gregory Schears, an associate professor of anesthesiology at the Mayo Clinic’s Pediatric Research Center in Minnesota. “Until now, there hasn’t been a good solution.”
There’s something on the horizon that may very well be.
For the past several years, a small Williamsburg, Va., company called ivWatch has been developing a device that would continuously monitor an IV and detect leaks almost immediately. The company is on track to seek approval from the Federal Drug Administration this fall, with the goal of having the product on the market early next year.
“We get calls all the time asking when we’re going be done,” says Gary Warren, the company’s chief executive officer.
Warren, a former NASA engineer who also headed several software companies, has led the ivWatch team since 2010, when the company was founded. The management team inherited intellectual property when they began ivWatch, along with 10 years of research that had already gone into the projeThe problem was, the product didn’t work. And it wasn’t discovered until after the company raised nearly $3 million from investors.
Despite the stumble, Warren and his team pressed on, spending about six months redesigning the product in order to put it through a trial at the Cincinnati Children’s Hospital Medical Center in Ohio, where they saw great results, Warren retells. Tried on 127 pediatric patients from Sept. 2011 to Feb. 2012, ivWatch was able to pick up 12 percent more infiltrations than the nurses noticed. On average, it detected a leak 21 hours before a nurse could, according to the company.
So what exactly is ivWatch?
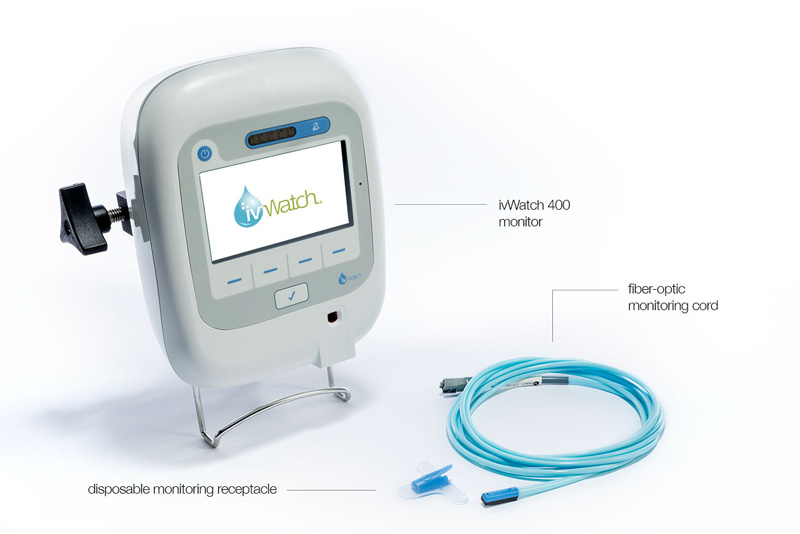
It’s a small, reusable module that uses a near-infrared light sensor to “see” what’s going on in the tissues. The module slips into a disposable receptacle that is attached to a patient’s skin right next to the IV needle. Tiny clips hold a flexible, fiber-optic cord to the IV tube.
The cord runs with the IV tube to the IV pole, where a monitoring device is clamped on over the box that controls the flow of saline or medication. If infiltration is suspected, a screen first flashes yellow, and then red to alert to a problem.
The average time an IV remains in a patient is 24 hours, according to Warren. He says an estimated one in six IVs goes bad. Ports and central lines are sometimes options, but they’re more expensive than IVs and drive up health-care costs,
says Dr. Brian Clare, ivWatch’s chief medical officer.
The company’s research found that the rate of IV infiltration in adults can be more than 10 percent, Warren explains. For children, and especially babies, the rate is much higher—more than 50 percent, possibly as high as 78 percent, according to the Infusion Nurses Society.
Pediatric patients, whose veins are more difficult to access because the vessels are smaller, usually aren’t able to tell a nurse if an IV doesn’t feel right, or if there’s swelling going on at the IV site, indicating fluid build-up. The same goes for elderly patients who might have altered mental states. So by the time the infiltration is realized, it can already have caused damage.
Besides the damage that infiltration might do, another big concern is that medication might not be getting where it needs to be, says Clare, a physician with a background in emergency medicine.
Even just simple saline that seeps into the arm tissues can cause serious issues. IvWatch’s executive summary, prepared for use during their various studies and trials, includes a photo of the arm of a 9-month-old baby who needed corrective surgery after a saline infiltration. The baby’s arm had to be cut from wrist to armpit to relieve pressure, and a skin graft was needed on the top of the hand.
“It’s one of the biggest health care problems that nobody talks about,” Warren says. “This technology has the chance to change all that.”
During a demonstration in ivWatch’s Williamsburg, Va., office building in McLaws Circle, it took under a minute for the device to detect IV infiltration in a pig’s foot. The company uses pig feet and ears for some of its testing because they have similar effects as infiltration in human forearms and hands.
The company has also done testing in its office—there’s a clinical room set up on the second floor—under the guidance of an investigational review board. The IRB reviews elements of the research and makes sure the rights and welfare of human subjects who take part are protected.
Warren suspects that the reason no one else has come up with a product like his is because of the time and energy it’s taken to bring it this far. When ivWatch, LLC, was founded in 2010, it took on the work that had been started with a grant from the National Institutes of Health in 2000.
Back then, the first sensor was made out of maple wood, which surprisingly had some element of success when tried on a volunteer group of University of Virginia students. Scientists later experimented with a plastic sensor, but that one didn’t work correctly, Warren says.
The rate of progress has skyrocketed in the past two years, he says. The newest design, which the ivWatch team affectionately calls “Gemini,” is what the team of scientists and medical experts—several of them former NASA employees—finally came up with. It’s the design the company will submit to the FDA when it asks for a validation study to make its claims.
The company has five manufacturers lined up to make the different components that go into the ivWatch device. The website, ivwatch.com, is set to go live soon. For months, visitors have only been able to glimpse at the logo and are told the site is under construction with a hint: “Actually, we are operating in stealth mode as we work on one of the biggest opportunities in health care.”
Schears, who chairs ivWatch’s advisory board and oversees the company’s clinical work, says he doesn’t expect any problem getting through the FDA. He calls the product a “no-risk technology.”
“It’s far superior to the standard,” Schears says. “Having seen what it can do, I really don’t think they’re going to have an issue.”
The company also intends to keep the cost down as much as possible. About 320 million IV sets are sold to medical facilities every year, and it costs about $45 a patient to put in a peripheral IV. IvWatch doesn’t want its sensor to add more than about
$5 per IV, in order to make it universally usable.
IvWatch should also help improve patient care by taking a big burden off nurses, says Clare. With the sensor to keep tabs on the IV, the nurses can focus more on the patient.
“We have a great opportunity to dramatically improve the level of care,” Schears says. “People will quickly realize the value in preventing these problems. I think it’ll be huge.”